Number mole relationship avogadro between avogadros moles concept chemistry molarity solute mass particles aplustopper into plus litre solution choose board Avogadro number mole moles stoichiometry chapter ppt powerpoint presentation formula mass acid calculate slideserve What is a mole and avogadro’s number
What is a Mole and Avogadro’s Number - YouTube
What is the relationship between a mole and avogadro's number The mole — definition & avogadro's number Avogadro number moles chapter formula na ppt powerpoint presentation
Using avogadro's number
Mole avogadroAvogadro avogadros experiment electrochemical refute Mole relationship number between avogadro mass particles avogadros other interconversion intoNumber mole chemistry avogadro avogadros get.
Avogadro’s numberElectrolytic determination of avogadro’s number Avogadro number mass mole between relation molecules chemistryAvogadro's number, the mole and how to use the mole.
Mole number avogadro avogadros chemistryworld
Avogadro atoms converting molesNumber using avogadro avogadros chemistry calculate atoms find flow diagram substance weebly Moles atoms mole au number gold molecules find many present chemistry mol avogadro mass socratic answer exactly case will formulasHow many atoms or molecules are present in 1.0 mol of au? how am i able.
Mole avogadro number use lifeChemistry lesson: the mole (avogadro's number) What is the relationship between a mole and avogadro's number.


Avogadro's Number, the Mole and How to Use the Mole - YouTube

Using Avogadro's Number - Chemistry Class 24/7
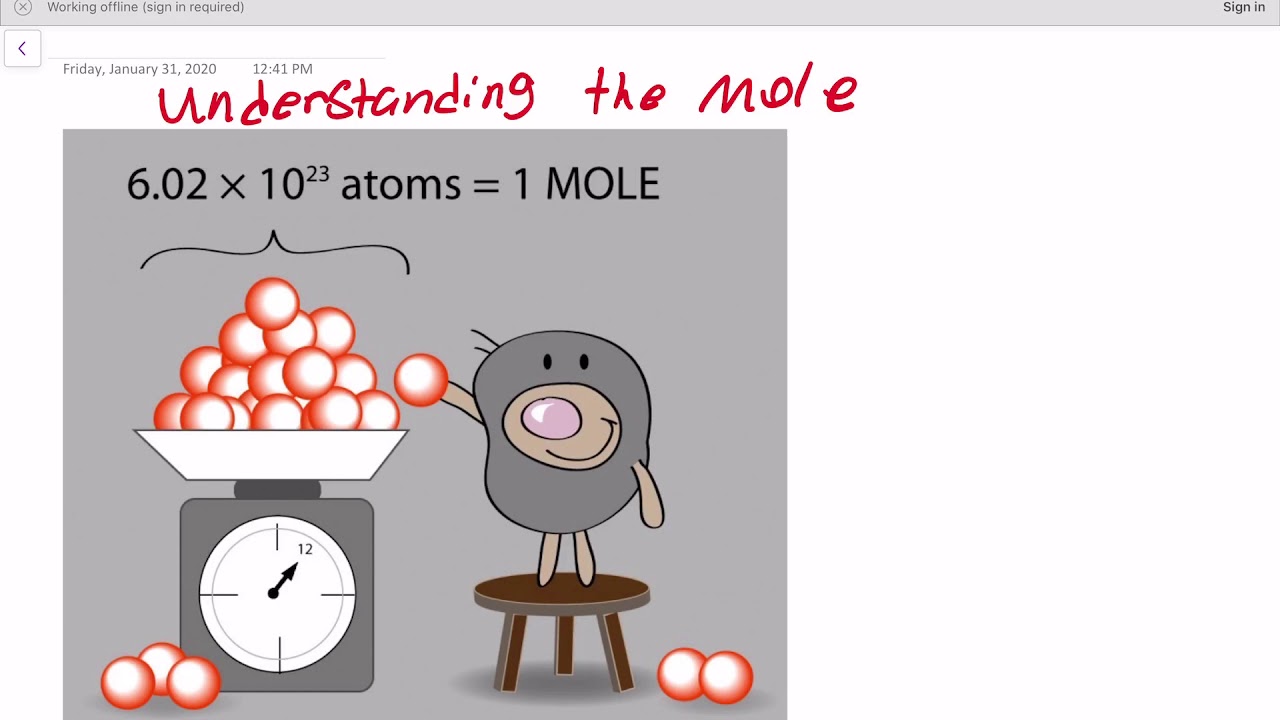
What is a Mole and Avogadro’s Number - YouTube
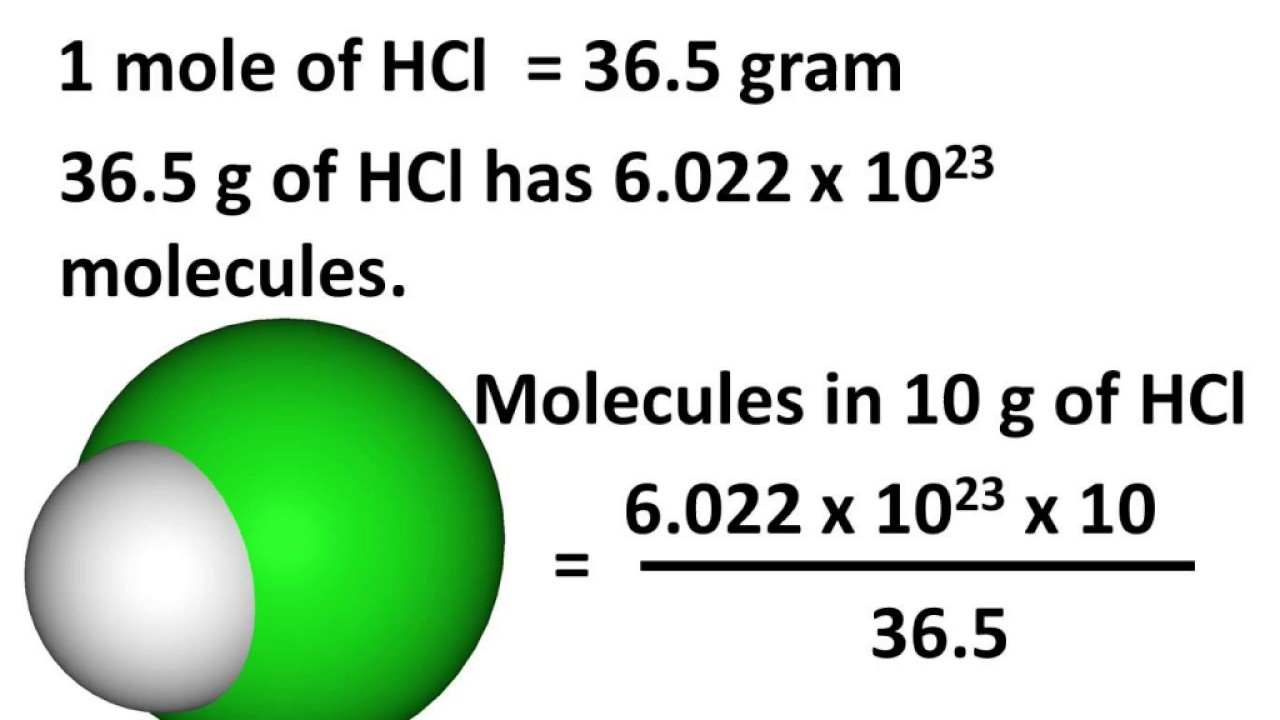
Chemistry - Relation between Mole, Avogadro number and Mass - Atoms and

PPT - Chapter 3 PowerPoint Presentation - ID:258640
.PNG)
Electrolytic Determination of Avogadro’s Number | Academic Brains Experts
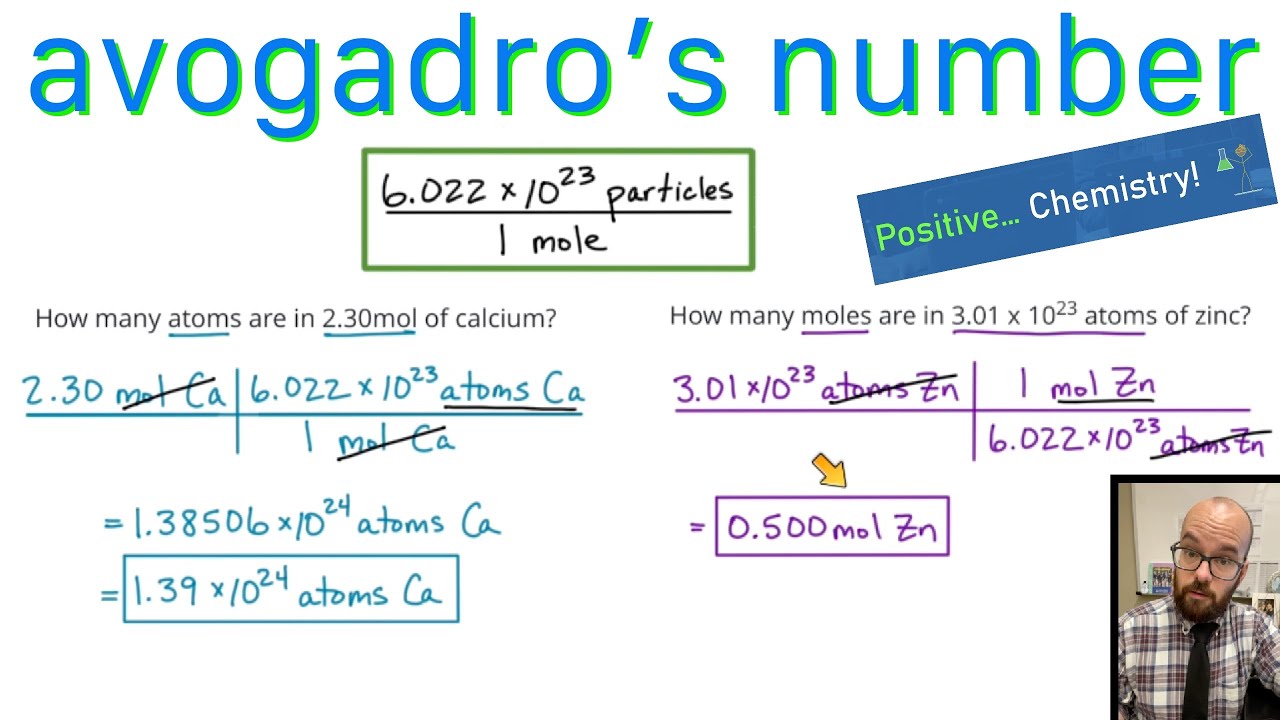
Avogadro’s Number - Converting between atoms and moles - YouTube

What is the Relationship between a Mole and Avogadro's number - A Plus

How many atoms or molecules are present in 1.0 mol of Au? How am I able

Chemistry Lesson: The Mole (Avogadro's Number) - Get Chemistry Help